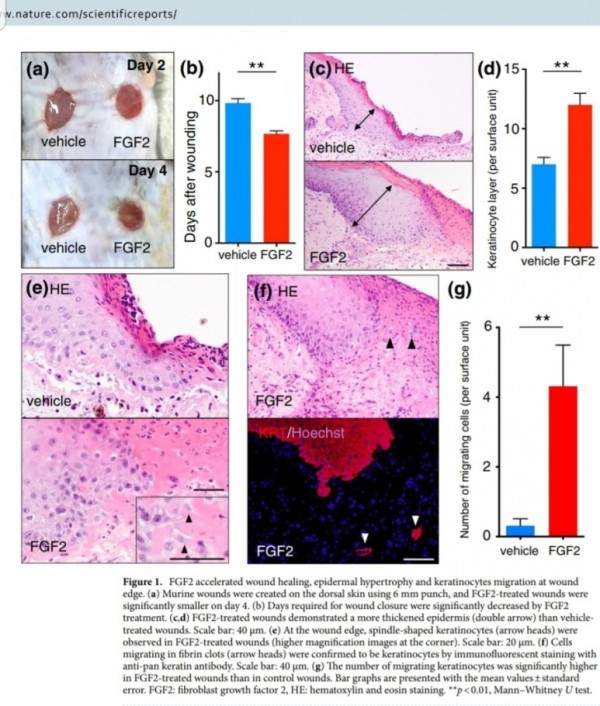
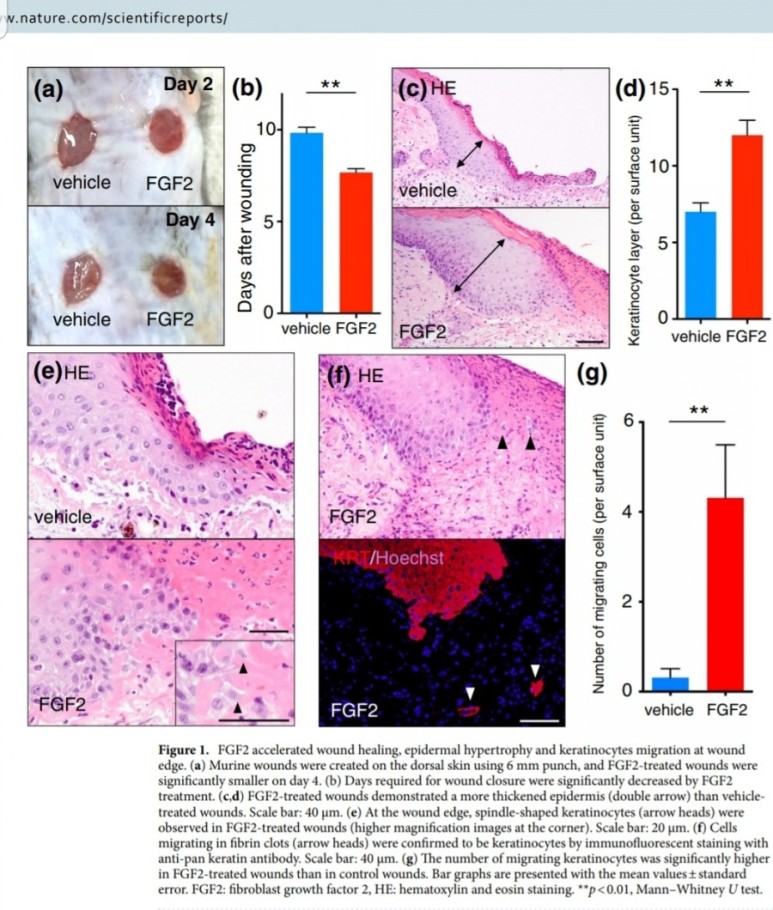
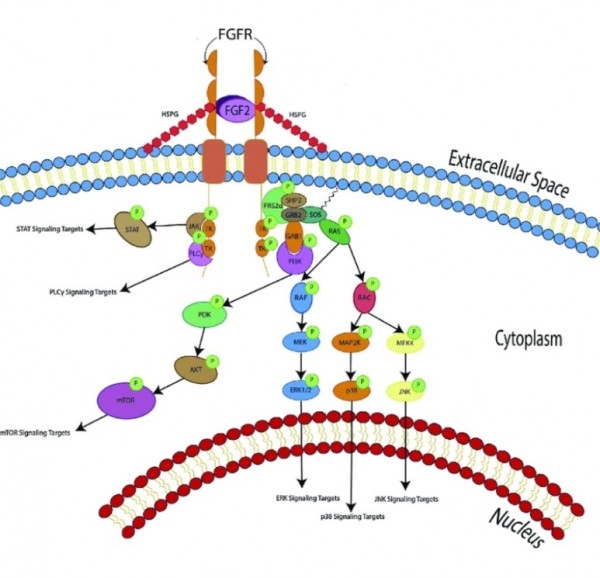
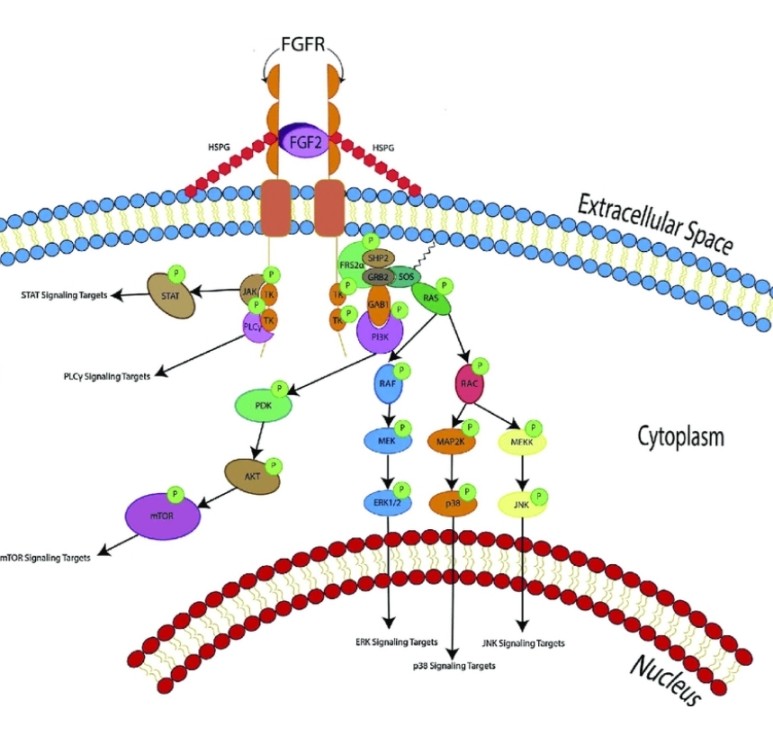
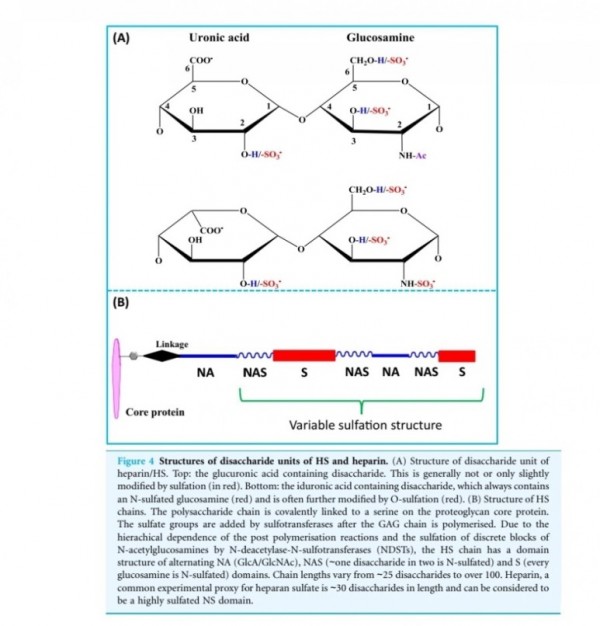
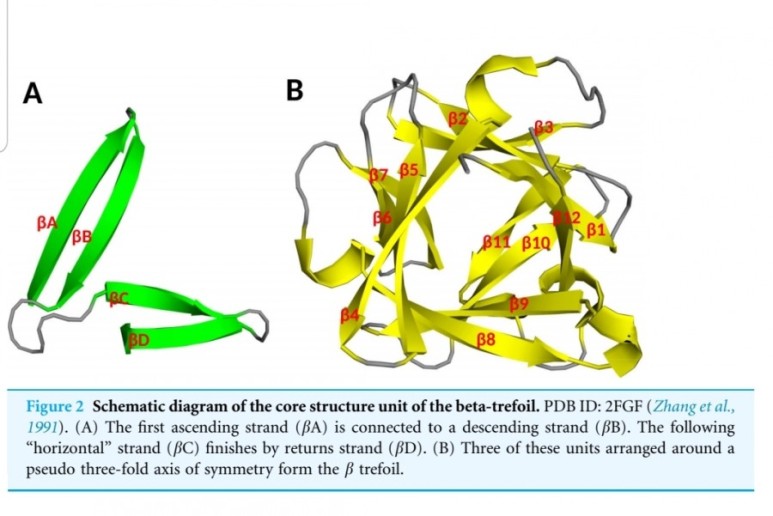
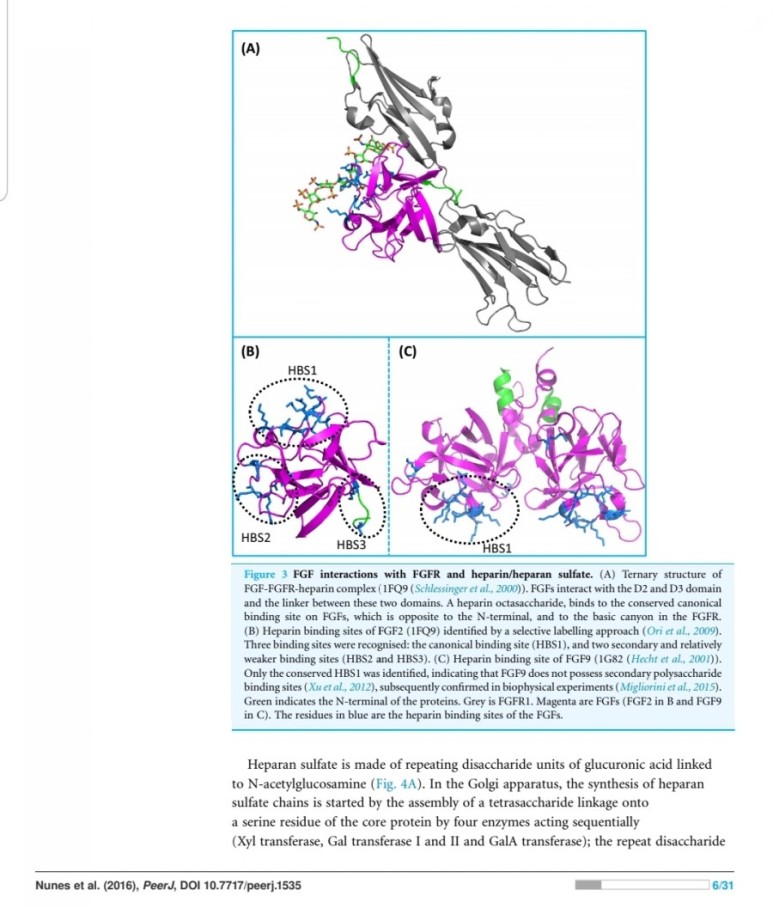
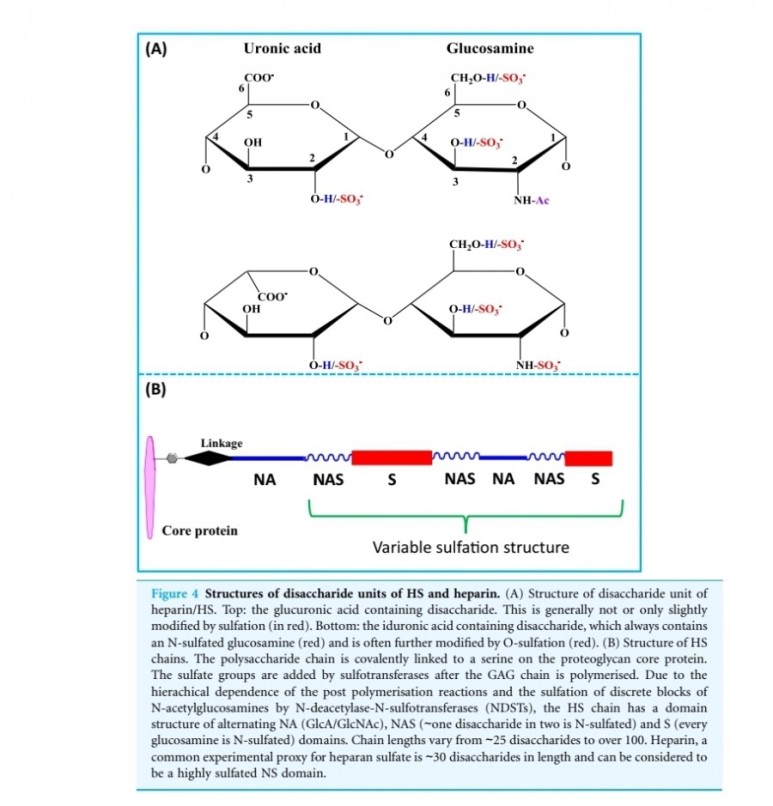
FGF2(basic fibroblast growth factor, bFGF)
페이지 정보
작성자 최고관리자 작성일 24-01-23 01:29 조회 5,614 댓글 0본문
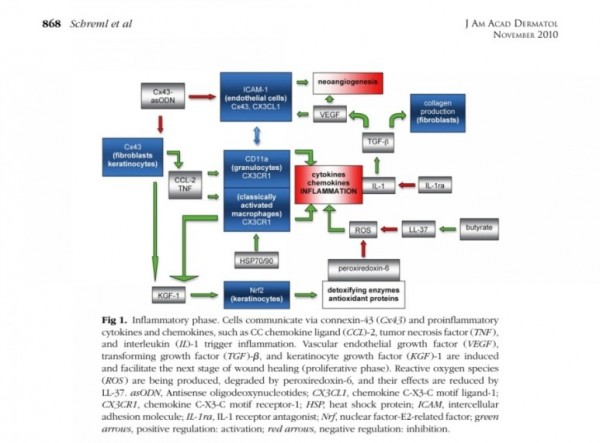
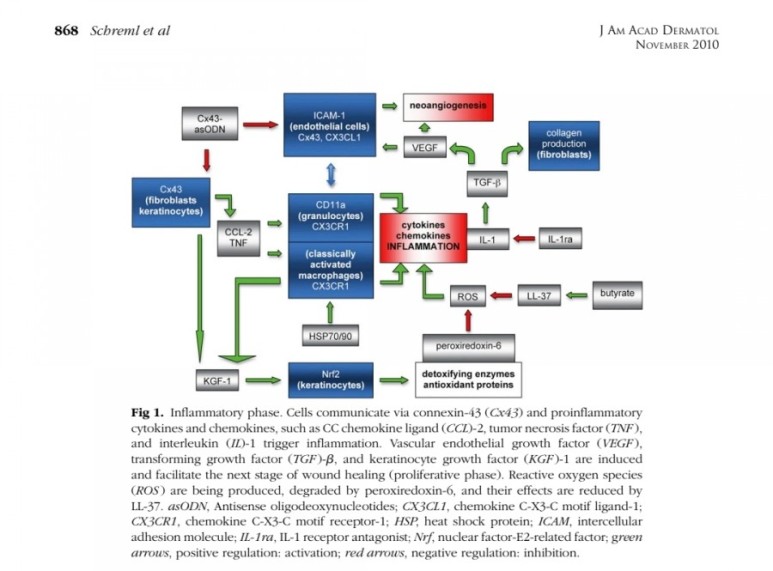
● 상처 재생 기능
표피생성 (epithelialization)
진피생성
혈관생성
※ 진피생성
collagen synthesis, ,
and fibronectin and proteoglycan synthesis
※ 발생학적 기능
태아발달 세포성장 형태형성
● FGF2를 생성하는 세포
fibroblast
endothelial cells
keratinocytes
vascular smooth muscle cells
neural cells
● 타겟 세포
표피생성위한 keratinocytes
진피생성위한 fibroblasts
혈관생성위한 endothelial cells
(capillary endothelial cells,
vascular endothelial cells,
fibroblasts,
keratinocytes,
epithelial cells and specialized cell types such as chondrocytes and myoblasts.)
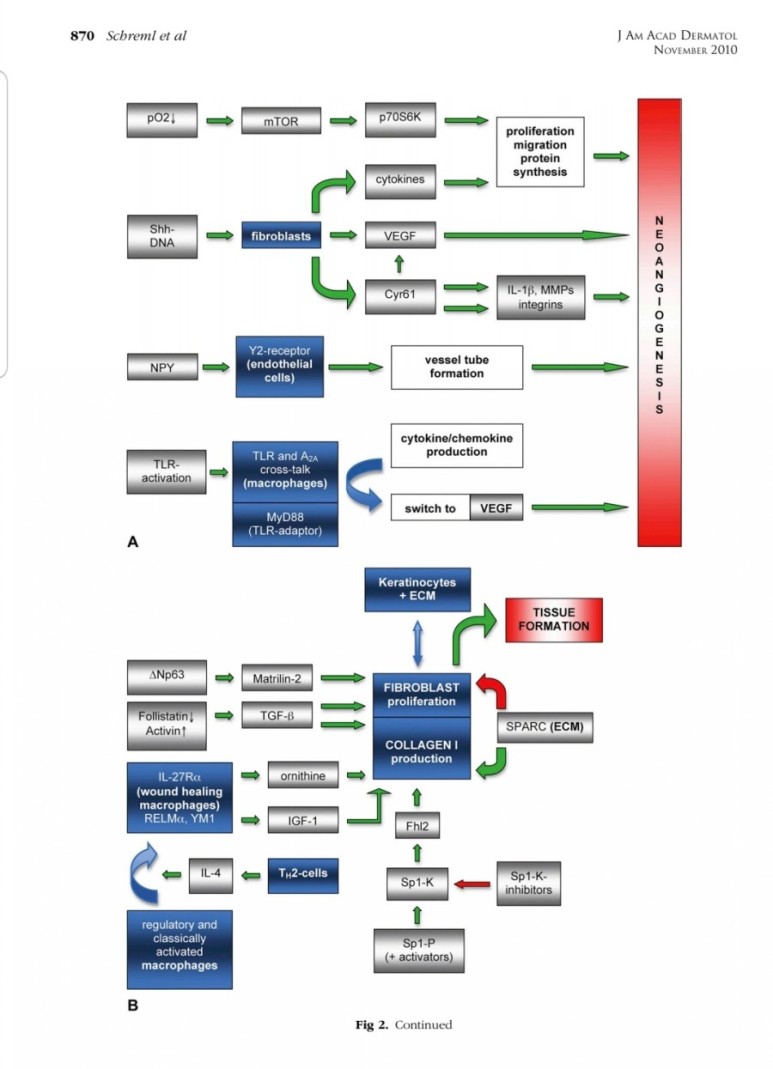
■ 상처회복시 기전
● fibroblast에서
fgf2 방출 : 상피화 (기저세포 이동)
콜라겐합성증가
혈관형성(angiogenesis)
fgf7, fgf10 방출 : 상피화(기저세포 증식)
● epidermal rdTC에서
fgf7, fgf10 방출 : 상피화(기저세포 증식)
※ 증식기
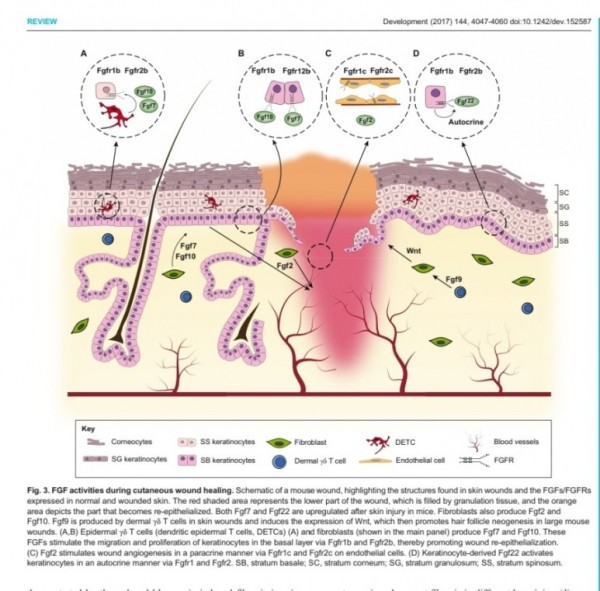
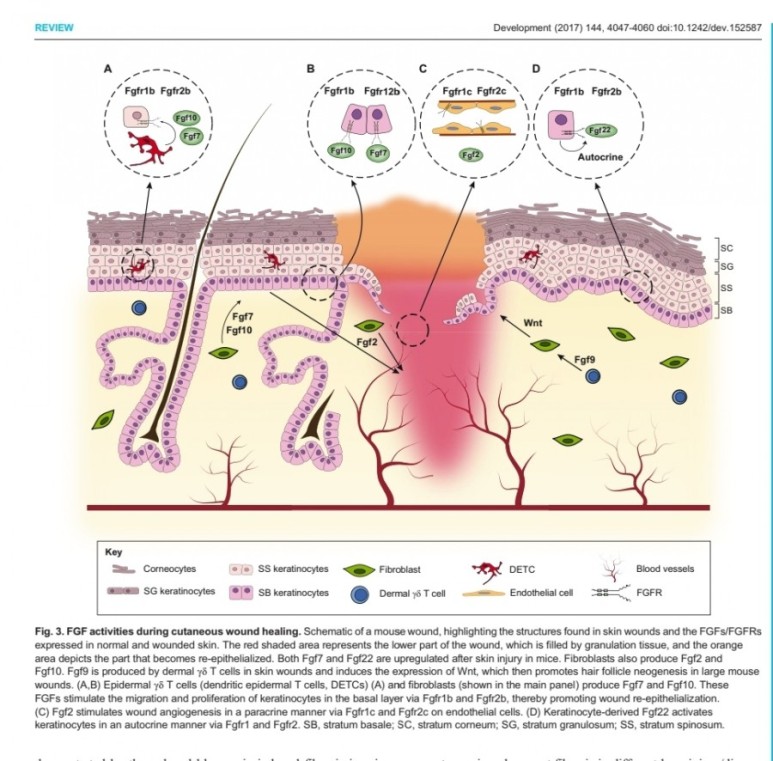
1. 표피재생(상피화)
- epidermal rdTC(dendritic T cell)과 fibroblast에서 fgf7, fgf10 방출 :
표피 기저세포 증식으로 상피화(nature 2017년)
- Fibroblast에서 방출한 fgf2가 epithelial–mesenchymal transition(EMT)을 촉진하여(mesenchymal epithelial cell로 분화) 상처부위로 이동하여 상피재생
(nature 2020년)
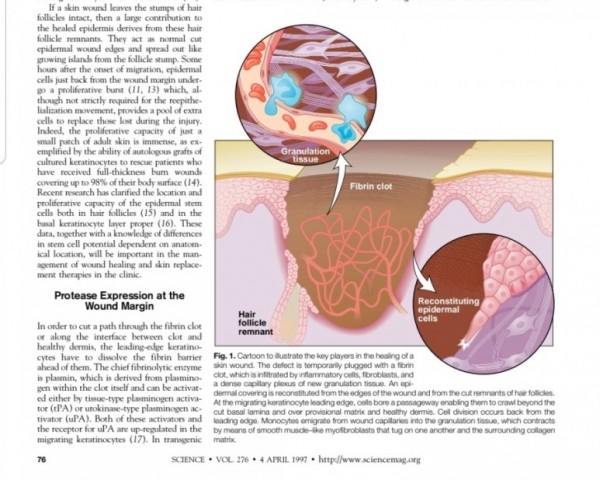
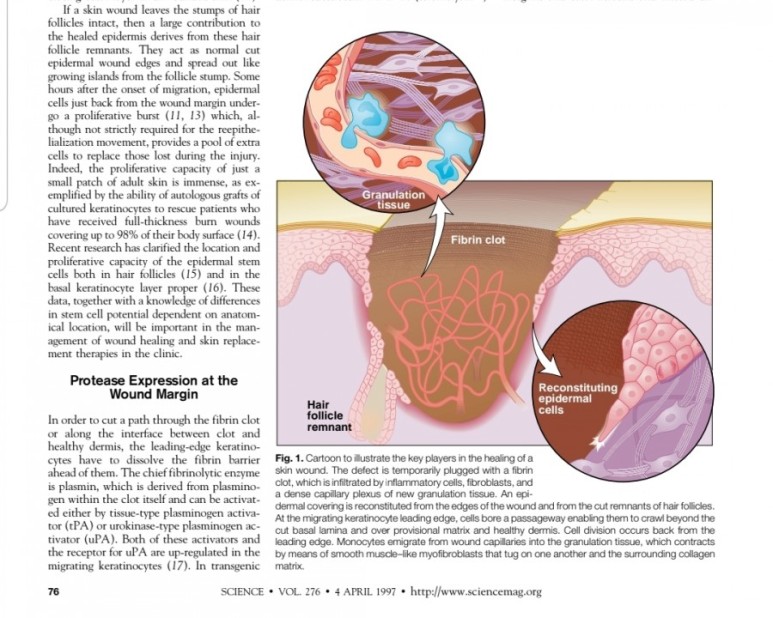
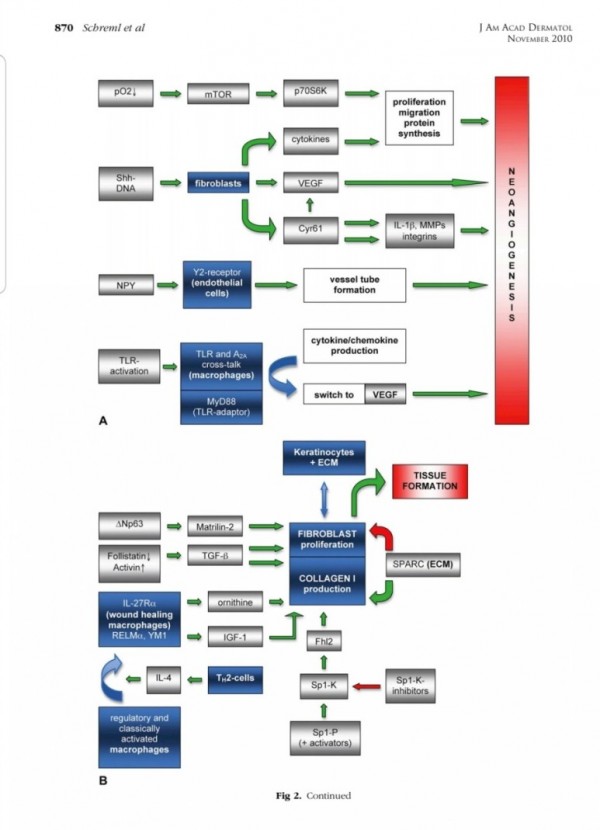
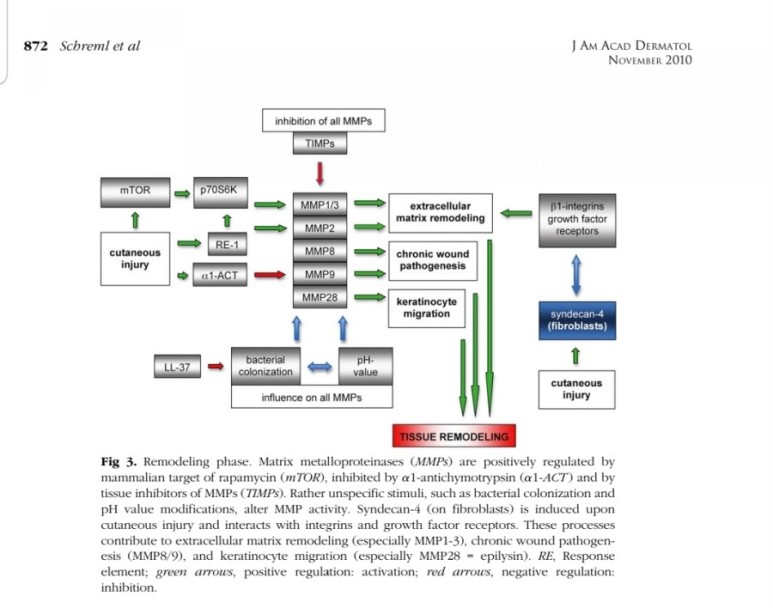
2. 진피재생
- 섬유아세포에서 분비된 fgf2가 주작용을 하다.
- 섬유아세포는 상처부위에서 근섬유아세포로 분화한다.
- 근섬유아세포는 세포외기질을 생성하여 육아조직형성한다.
(육아조직은 섬유와 혈관으로 구성)
3. 혈관재생
내막세포는 성장인자에 의해 활성화되고
혈관신생을 한다.
표피재생에 FGF10(KGF2)
진피의 재생에 FGF2 가 중요하다.
※ 사이언스 1997년 논문
※ 기타
# 지방세포도 FGF2를 생성하고 분비한다.
# FGF2는 심근경색시 심장손상을 억제하고 혈관재생을 도와 조직의 괴사를 줄인다.
# FGF2가 부족하면 과도한 화를 잘낸다는 보고도 있다.
# 아래는 wikipedia 펀글임
FGF2, also known as basic fibroblast growth factor (bFGF) and FGF-β, is a growth factor and signaling protein encoded by the FGF2 gene.[5][6]
It is synthesized primarily as a 155 amino acid polypeptide, resulting in an 18 kDa protein. However, there are four alternate start codons which provide N-terminal extensions of 41, 46, 55, or 133 amino acids, resulting in proteins of 22 kDa (196 aa total), 22.5 kDa (201 aa total), 24 kDa (210 aa total) and 34 kDa (288 aa total), respectively.[7] Generally, the 155 aa/18 kDa low molecular weight (LMW) form is considered cytoplasmic and can be secreted from the cell, whereas the high molecular weight (HMW) forms are directed to the cell's nucleus.[8]
Fibroblast growth factor protein was first purified in 1975, but soon afterwards others using different conditions isolated basic FGF, Heparin-binding growth factor-2, and Endothelial cell growth factor-2.
Gene sequencing revealed that this group was in fact the same FGF2 protein and that it was a member of a family of FGF proteins.[7][9]
FGF2 binds to and exerts effects via specific fibroblast growth factor receptor (FGFR) proteins which themselves constitute a family of closely related molecules.
Like other FGF family members,
basic fibroblast growth factor possess
broad mitogenic
and cell survival activities,
and is involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumor growth and invasion.
In normal tissue, bFGF is present in basement membranes and in the subendothelial extracellular matrix of blood vessels.
It stays membrane-bound as long as there is no signal peptide.
It has been hypothesized that, during both wound healing of normal tissues and tumor development, the action of heparan sulfate-degrading enzymes activates bFGF, thus mediating the formation of new blood vessels, a process known as angiogenesis.
In addition, it is synthesized and secreted by human adipocytes and the concentration of FGF2 correlates with the BMI in blood samples.
It was also shown to act on preosteoblasts – in the form of an increased proliferation – after binding to fibroblast growth factor receptor 1 and activating phosphoinositide 3-kinase.[10]
FGF2 has been shown in preliminary animal studies to protect the heart from injury associated with a heart attack, reducing tissue death and promoting improved function after reperfusion.[11]
Recent evidence has shown that low levels of FGF2 play a key role in the incidence of excessive anxiety.[12]
Additionally, FGF2 is a critical component of human embryonic stem cell culture medium; the growth factor is necessary for the cells to remain in an undifferentiated state, although the mechanisms by which it does this are poorly defined. It has been demonstrated to induce gremlin expression which in turn is known to inhibit the induction of differentiation by bone morphogenetic proteins.[13]
It is necessary in mouse-feeder cell dependent culture systems, as well as in feeder and serum-free culture systems.[14]
FGF2, in conjunction with BMP4, promote differentiation of stem cells to mesodermal lineages.
After differentiation, BMP4 and FGF2 treated cells generally produce higher amounts of osteogenic and chondrogenic differentiation than untreated stem cells.[15]
However, a low concentration of bFGF (10 ng/mL) may exert an inhibitory effect on osteoblast differentiation.[16] The nuclear form of FGF2 functions in mRNA export[17]
Interactions[edit]
Basic fibroblast growth factor has been shown to interact with casein kinase 2, alpha 1,[18] RPL6,[19] ribosomal protein S19[20] and API5.[17]
See also
References
^ Jump up to:a b c GRCh38: Ensembl release 89: ENSG00000138685 - Ensembl, May 2017
^ Jump up to:a b c GRCm38: Ensembl release 89: ENSMUSG00000037225 - Ensembl, May 2017
^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
^ Dionne CA, Crumley G, Bellot F, Kaplow JM, Searfoss G, Ruta M, Burgess WH, Jaye M, Schlessinger J (September 1990). "Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors". The EMBO Journal. 9 (9): 2685–92. doi:10.1002/j.1460-2075.1990.tb07454.x. PMC 551973. PMID 1697263.
^ Kim HS (1998). "Assignment1 of the human basic fibroblast growth factor gene FGF2 to chromosome 4 band q26 by radiation hybrid mapping". Cytogenetics and Cell Genetics. 83 (1–2): 73. doi:10.1159/000015129. PMID 9925931. S2CID 33214466.
^ Jump up to:a b Florkiewicz RZ, Shibata F, Barankiewicz T, Baird A, Gonzalez AM, Florkiewicz E, Shah N (December 1991). "Basic fibroblast growth factor gene expression". Annals of the New York Academy of Sciences. 638 (1): 109–26. doi:10.1111/j.1749-6632.1991.tb49022.x. PMID 1785797. S2CID 45425517.
^ Coleman SJ, Bruce C, Chioni AM, Kocher HM, Grose RP (August 2014). "The ins and outs of fibroblast growth factor receptor signalling". Clinical Science. 127 (4): 217–31. doi:10.1042/CS20140100. PMID 24780002.
^ Burgess WH, Maciag T (1989). "The heparin-binding (fibroblast) growth factor family of proteins". Annual Review of Biochemistry. 58: 575–606. doi:10.1146/annurev.bi.58.070189.003043. PMID 2549857.
^ Kühn MC, Willenberg HS, Schott M, Papewalis C, Stumpf U, Flohé S, Scherbaum WA, Schinner S (February 2012). "Adipocyte-secreted factors increase osteoblast proliferation and the OPG/RANKL ratio to influence osteoclast formation". Molecular and Cellular Endocrinology. 349 (2): 180–8. doi:10.1016/j.mce.2011.10.018. PMID 22040599. S2CID 2305986.
^ House SL, Bolte C, Zhou M, Doetschman T, Klevitsky R, Newman G, Schultz Jel J (December 2003). "Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia". Circulation. 108 (25): 3140–8. doi:10.1161/01.CIR.0000105723.91637.1C. PMID 14656920. S2CID 14251918.
^ Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H (May 2009). "A new role for FGF2 as an endogenous inhibitor of anxiety". The Journal of Neuroscience. 29 (19): 6379–87. doi:10.1523/JNEUROSCI.4829-08.2009. PMC 2748795. PMID 19439615.
^ Pereira RC, Economides AN, Canalis E (December 2000). "Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts". Endocrinology. 141 (12): 4558–63. doi:10.1210/en.141.12.4558. PMID 11108268. Archived from the original on 2012-07-11.
^ Liu Y, Song Z, Zhao Y, Qin H, Cai J, Zhang H, Yu T, Jiang S, Wang G, Ding M, Deng H (July 2006). "A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells". Biochemical and Biophysical Research Communications. 346 (1): 131–9. doi:10.1016/j.bbrc.2006.05.086. PMID 16753134.
^ Lee TJ, Jang J, Kang S, Jin M, Shin H, Kim DW, Kim BS (January 2013). "Enhancement of osteogenic and chondrogenic differentiation of human embryonic stem cells by mesodermal lineage induction with BMP-4 and FGF2 treatment". Biochemical and Biophysical Research Communications. 430 (2): 793–7. doi:10.1016/j.bbrc.2012.11.067. PMID 23206696.
^ Del Angel-Mosqueda C, Gutiérrez-Puente Y, López-Lozano AP, Romero-Zavaleta RE, Mendiola-Jiménez A, Medina-De la Garza CE, Márquez-M M, De la Garza-Ramos MA (September 2015). "Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro". Head & Face Medicine. 11: 29. doi:10.1186/s13005-015-0086-5. PMC 4558932. PMID 26334535.
^ Jump up to:a b Bong SM, Bae SH, Song B, Gwak H, Yang SW, Kim S, Nam S, Rajalingam K, Oh SJ, Kim TW, Park S, Jang H, Lee BI (June 2020). "Regulation of mRNA Export Through API5 and Nuclear FGF2 Interaction". Nucleic Acids Research. 48 (11): 6340–6352. doi:10.1093/nar/gkaa335. PMC 7293033. PMID 32383752.
^ Skjerpen CS, Nilsen T, Wesche J, Olsnes S (August 2002). "Binding of FGF-1 variants to protein kinase CK2 correlates with mitogenicity". The EMBO Journal. 21 (15): 4058–69. doi:10.1093/emboj/cdf402. PMC 126148. PMID 12145206.
^ Shen B, Arese M, Gualandris A, Rifkin DB (November 1998). "Intracellular association of FGF-2 with the ribosomal protein L6/TAXREB107". Biochemical and Biophysical Research Communications. 252 (2): 524–8. doi:10.1006/bbrc.1998.9677. PMID 9826564.
^ Soulet F, Al Saati T, Roga S, Amalric F, Bouche G (November 2001). "Fibroblast growth factor-2 interacts with free ribosomal protein S19". Biochemical and Biophysical Research Communications. 289 (2): 591–6. doi:10.1006/bbrc.2001.5960. PMID 11716516.
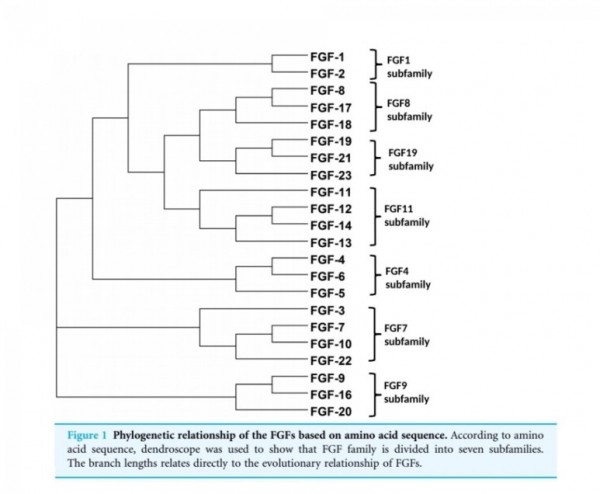
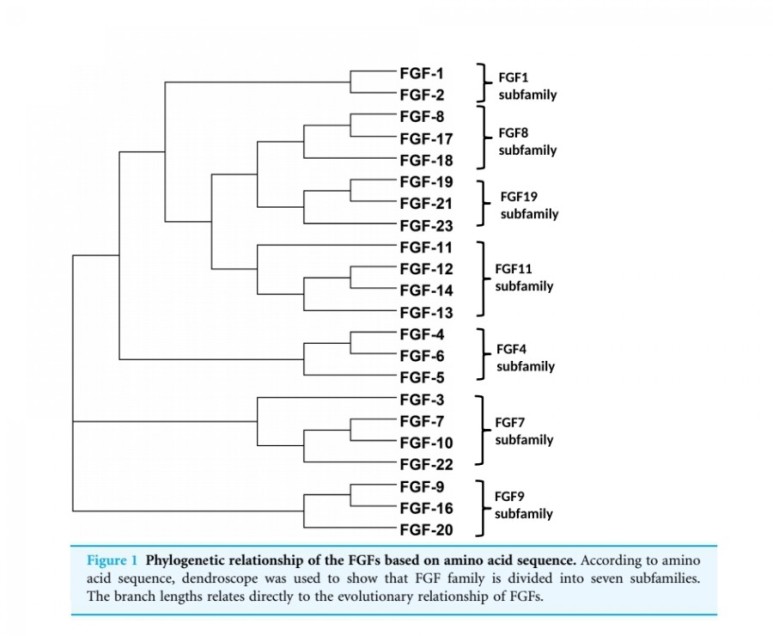
■ FGF2
Fibroblast growth factors
The fibroblast growth factor (FGF) family
comprises several related polypeptides, including
acidic FGF (aFGF or FGF-1),
basic FGF (bFGF or FGF-2),
several oncogenes (int-2, hst/K-FGF, FGF-5)
and KGF (54–60).
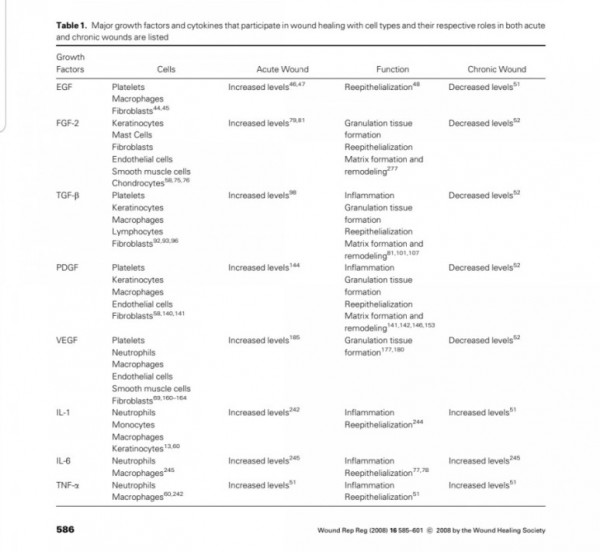
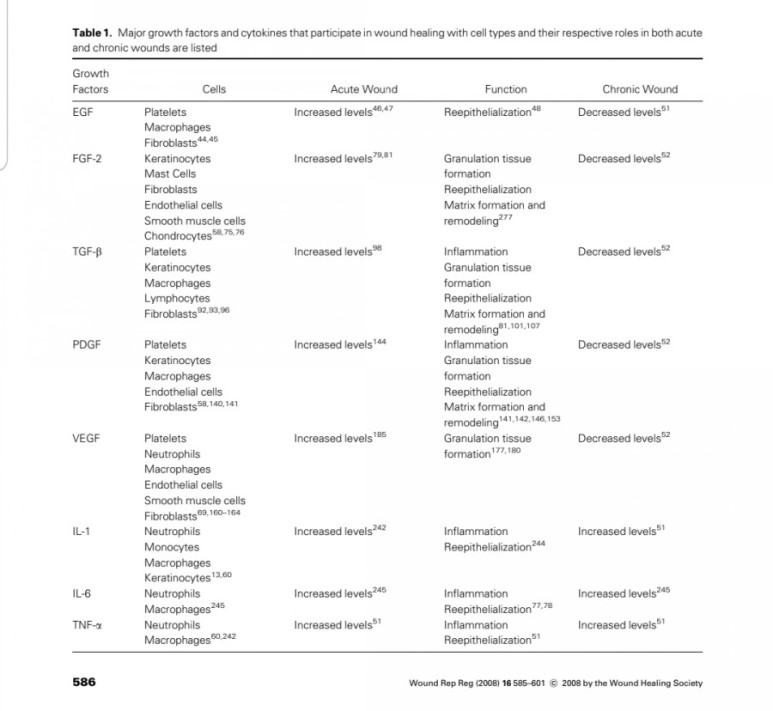
Three proteins of the FGF family are thought to be important regulators of wound healing:
FGF-1, FGF-2 and KGF (1).
Although FGF-1 and FGF-2 are two separate
entities, they both have a strong affinity for the glycosaminoglycan heparin sulfate (9).
Thus, they are not freely soluble molecules but are associated with the extracellular matrix.
Both FGF-1 and FGF-2 are
potent mitogens for cells derived from mesoderm
and neuroectoderm, and they share
many biochemical and biological properties.
They are both single-chain proteins (140 and 146 amino acids,respectively) while their names reflect their isoelectric points of pH 5.6 and pH 9.6.
FGF-1 and FGF-2 have 50% amino-acid homology, though FGF-2 is approximately 10 times more potent as an angiogenic stimulant (11).
Of all known growth factors,
FGF-2 probably has the broadest range of
target cells, including nearly all of the diverse cell
types involved in wound healing (61).
Both FGF-1 and FGF-2 have been isolated from
a variety of cells (11). The cells producing FGF in-
clude endothelial cells, vascular smooth muscle
cells, neural cells and keratinocytes, and FGFs are
widely distributed throughout most organ tissues
(11, 62).
A specific surface receptor for FGFs is
found on most cells of ectodermal or mesodermal
origin. Paradoxically, no secretory mechanism has
been described for FGFs, suggesting that native
FGFs may be primarily cell-associated (62).
FGFs stimulate the proliferation and/or migration
both in vitro and in vivo of most of the major cell
types involved in wound healing, including capillary endothelial cells, vascular endothelial cells, fibroblasts, keratinocytes, epithelial cells and specialized cell types such as chondrocytes and myoblasts (1,60, 63, 64).
FGF-2 also stimulates collagen synthesis, epithelialization, and fibronectin and proteo-
glycan synthesis (11).
In addition, FGF-2 induces
cell migration, neovascularization and formation of granulation tissue in animal models (1, 2, 60).
Although FGF-1 and FGF-2 are ubiquitous growth
factors that stimulate proliferation and differentia-tion of a wide variety of adult and embryonic cell
types, they are also potent angiogenic factors (59,
65, 66). Since FGFs probably play a mediatory role
in the angiogenic process in a variety of tissues,
and angiogenesis occurs normally during tissue re-pair such as in healing wounds and fractures, these two growth factors have been evaluated in most studies of healing tissues (8, 63). Studies using the mouse gene-knockout model have demonstrated that
lack of FGF-2 expression causes delayed wound
healing (67).
Not surprisingly, both FGF-1 and FGF-2 stimu-
late endothelial cell proliferation and thus contri-
bute to wound neovascularization, and their activity is potentiated by heparin (22).
FGFs stimulate endothelial migration, proliferation and capillary-like tube formation, promoting new vessel growth in vivo and in vitro (62).
In surgical incisions, Nissen et al. (68) have
found high levels of FGF-2 that are directly corre-
lated with an increased endothelial cell prolifera-
tion. The direct application of FGF to wounds ac-
celerates healing in both linear incisions and ulcer
models (69). A positive influence of recombinant
human FGF-2 on healing wounds has also been
observed (60).
In addition, FGF-2 has been used to stimulate endothelial population of vascular pros-
theses (62).
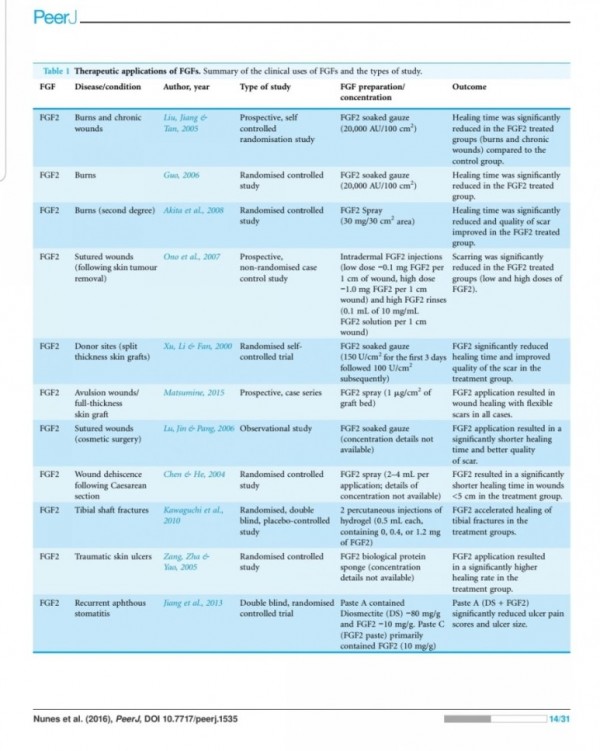
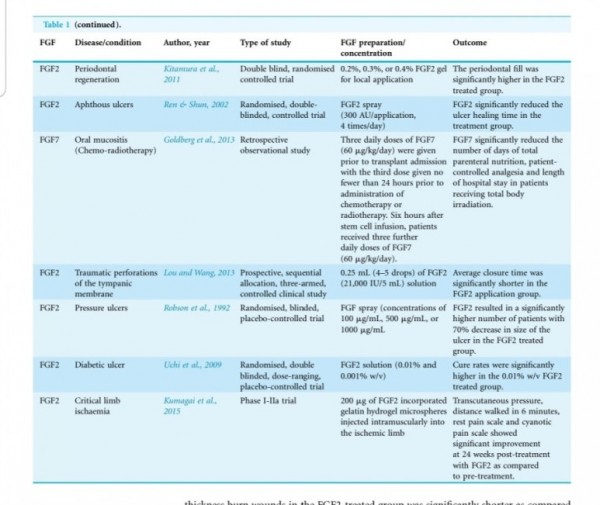
In animal systems, FGF-2 also appears to stimulate the healing of partial- and full- thickness wounds of the skin, cornea, cartilage and brain, while FGF-1 stimulates the repair of peripheral nerves (62).
The local application of FGF also accelerates
wound healing in patients with second-degree
burns and chronic dermal ulcers by the accelera-
tion of the rate of granulation tissue formation and epidermal regeneration as compared with that in the controls (70, 71). Moreover, FGF-2 also increases the breaking strength of acute incisional wounds (72).
The administration of FGF-2 at the
time of wound closure not only significantly in-
creases the breaking strength of the wound, but also improves the quality of the scar (72, 73).
#닥터오라클 #오라클코스메틱
#오라클피부과 #droracle #oracle_cosmetic
#FGF2 #Fibroblast_Growth_Factor2
#basic_Fibroblast_Growth_Factor
#bFGF
댓글목록 0
등록된 댓글이 없습니다.